
-
 Bourgeois inspires France to Six Nations rout of Scots
Bourgeois inspires France to Six Nations rout of Scots
-
Sudan army says retakes Khartoum-area market from paramilitaries

-
 Eze leads Crystal Palace into FA Cup semi-finals
Eze leads Crystal Palace into FA Cup semi-finals
-
Guinea ex-dictator freed from jail after 2009 massacre pardon: junta

-
 Martinez punishment 'out of Flick's hands' as Barca focus on title
Martinez punishment 'out of Flick's hands' as Barca focus on title
-
Hundreds of thousands join Istanbul protest rally

-
 Australian sprinting prodigy Gout Gout upstaged in 200m
Australian sprinting prodigy Gout Gout upstaged in 200m
-
'We need aid': rescuers in quake-hit Myanmar city plead for help

-
 Protesters flock to mass opposition rally in Istanbul
Protesters flock to mass opposition rally in Istanbul
-
Are women allowed their own dreams, wonders Chimamanda Ngozi Adichie

-
 Deadly earthquake forces Thai patients into sports hall
Deadly earthquake forces Thai patients into sports hall
-
'Everyone was screaming': quake shocks Thailand tourists

-
 Rallies grow in South Korea as court weighs president's fate
Rallies grow in South Korea as court weighs president's fate
-
Scientists explain why Myanmar quake was so deadly

-
 Turkey opposition calls mass rally in Istanbul
Turkey opposition calls mass rally in Istanbul
-
Chapman blasts ton as New Zealand win first Pakistan ODI by 73 runs

-
 French chefs quake as Michelin prepares new guide
French chefs quake as Michelin prepares new guide
-
Mike Leigh on the 'hard truths' of film, happiness and World War III

-
 Myanmar quake toll passes 1,000 as rescuers dig for survivors
Myanmar quake toll passes 1,000 as rescuers dig for survivors
-
Lights out: Bali guards protect island's day of silence

-
 Myanmar-Thailand quake toll passes 700 as rescuers dig for survivors
Myanmar-Thailand quake toll passes 700 as rescuers dig for survivors
-
UK gallery to return Nazi-looted painting to heirs of Jewish collector

-
 UK dreams of US trade deal before Trump tariffs
UK dreams of US trade deal before Trump tariffs
-
'Blink of an eye': survivor tells of Bangkok skyscraper collapse horror

-
 The hand of GOAT, Mensik wins with Messi touch
The hand of GOAT, Mensik wins with Messi touch
-
Partial solar eclipse to cross swathe of Northern Hemisphere

-
 Tunisian startup turns olive waste into clean energy
Tunisian startup turns olive waste into clean energy
-
Guinea ex-dictator sentenced for 2009 massacre pardoned: junta

-
 Chapman ton lifts New Zealand to 344-9 in first Pakistan ODI
Chapman ton lifts New Zealand to 344-9 in first Pakistan ODI
-
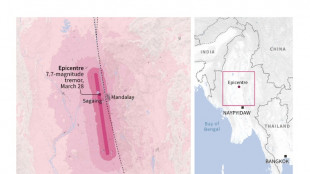
Myanmar quake: what we know
-
 Vu fires 64 to seize lead at LPGA Ford Championship
Vu fires 64 to seize lead at LPGA Ford Championship
-
Resurgent Liu wins women's figure skating world title

-
 Djokovic to face Mensik with 100th title within reach
Djokovic to face Mensik with 100th title within reach
-
Rescuers dig for survivors after huge quake hits Myanmar, Thailand

-
 South Korea firefighters deploy helicopters as wildfires reignite
South Korea firefighters deploy helicopters as wildfires reignite
-
'Defiant' Canada autoworkers vow to fight tariff layoffs

-
 Performance, museums, history: Trump's cultural power grab
Performance, museums, history: Trump's cultural power grab
-
Russian-born 12-ranked Kasatkina says to play for Australia tennis

-
 WyHy Celebrates Grand Opening of New Lyman Branch
WyHy Celebrates Grand Opening of New Lyman Branch
-
New to The Street Show #639 Premieres Nationwide on Bloomberg Television, March 29 at 6:30 PM EST Featuring BioVie, Roadzen, Tonix Pharma, and eXoZymes Inc.

-
 Wallabies back Jorgensen suffers serious ankle injury
Wallabies back Jorgensen suffers serious ankle injury
-
Academy apologizes after stars say it 'failed to defend' Palestinian filmmaker

-
 UN rights chief demands end to 'horrific suffering' in Ukraine
UN rights chief demands end to 'horrific suffering' in Ukraine
-
Djokovic oozing confidence ahead of century bid

-
 US regulators to investigate Disney diversity efforts
US regulators to investigate Disney diversity efforts
-
Elon Musk says xAI startup buying X platform

-
 'Jail or death': migrants expelled by Trump fear for their fate
'Jail or death': migrants expelled by Trump fear for their fate
-
Djokovic closing in on 100th title after Dimitrov downed in Miami

-
 Leverkusen beat Bochum to stay hot on Bayern's heels
Leverkusen beat Bochum to stay hot on Bayern's heels
-
Global markets slide as fears over US tariffs intensify


IGC Pharma's Phase 2 Clinical Trial Interim Data Demonstrates Significant Reduction in Sleep Disturbances
- IGC-AD1 Could Offer Safer Alternative to Existing Sleep Medications for Alzheimer’s -
POTOMAC, MD / ACCESS Newswire / March 26, 2025 / IGC Pharma, Inc. (NYSE American:IGC) ("IGC Pharma" or the "Company") today announced additional positive interim results from its ongoing Phase 2 clinical trial on IGC-AD1, an investigational treatment for agitation in dementia due to Alzheimer's disease.

Based on the interim analysis at week 2 sleep disturbance was reduced by about 71% (p=.012) and at week 6 about 78% (p=.02) for those on the active medication. These values indicate a clinical and statistically significant reduction in sleep disturbances among Alzheimer's patients receiving the active medication compared to placebo, as measured by the Neuropsychiatric Inventory ("NPI-12") Sleep Subscale.
The results suggest that IGC-AD1 may decrease the frequency and/or severity of sleep disturbances and nighttime behaviors, addressing a critical yet underrecognized challenge in Alzheimer's care that impacts up to 44% of Alzheimer's patients.
"IGC-AD1's ability to improve sleep quality in Alzheimer's patients is very exciting," said Ram Mukunda, CEO of IGC Pharma. "Better sleep is linked to reduced agitation and caregiver distress, as well as slowing cognitive decline and improving overall quality of life."
Improved sleep quality has been linked to reduced amyloid accumulation and slower disease progression. Additionally, previously reported data from the trial demonstrated notable reductions in agitation, reinforcing IGC-AD1's potential as a multi-targeted therapy for addressing neuropsychiatric symptoms in Alzheimer's patients.
Current treatment options including Trazodone and Suvorexant have shown limited improvement in placebo-controlled trials with dementia patients. IGC-AD1's differentiated mechanism offers the potential, if confirmed by larger trials, for a safer, more effective alternative for Alzheimer's patients.
Beyond Alzheimer's over 30 million Americans suffer from sleep disorders, which is a risk factor for cognitive decline and cardiovascular disease. The global sleep aid market is projected to surpass $100 billion by 2030, presenting, if verified through larger trials, a significant commercial opportunity for innovative therapies like IGC-AD1.
IGC-AD1 is a cannabinoid-based partial CB1 receptor agonist with anti-neuroinflammatory and neuroprotective properties. Unlike sedatives, which primarily mask symptoms, IGC-AD1 may, if confirmed by larger trials, provide a safer, more effective approach to target sleep regulation in Alzheimer's disease.
IGC Pharma is progressing its Phase 2 trial of IGC-AD1, recently named CALMA, with further analysis expected in the end-2025, including on sleep disturbance. The Company also expects to launch studies to evaluate IGC-AD1 as an Alzheimer's disease modifying drug.
For more information about the ongoing clinical trial, visit clinicaltrials.gov.
About IGC Pharma (dba IGC):
IGC Pharma (NYSE American:IGC) is a clinical-stage biotechnology company leveraging AI to develop innovative treatments for Alzheimer's and metabolic disorders. Our lead asset, IGC-AD1, is a cannabinoid-based therapy currently in a Phase 2 trial (CALMA) for agitation in Alzheimer's dementia. Our pipeline includes TGR-63, targeting amyloid plaques, and early-stage programs focused on neurodegeneration, tau proteins, and metabolic dysfunctions. We integrate AI to accelerate drug discovery, optimize clinical trials, and enhance patient targeting. With 55 patent filings and a commitment to innovation, IGC Pharma is advancing breakthrough therapies. Additionally, the Company operates Holiby™, a wellness brand offering scientifically formulated products for immunity, energy, and longevity.
Forward-Looking Statements:
This press release contains forward-looking statements. These forward-looking statements are based largely on IGC Pharma's expectations and are subject to several risks and uncertainties, certain of which are beyond IGC Pharma's control. Actual results could differ materially from these forward-looking statements as a result of, among other factors, the Company's failure or inability to commercialize one or more of the Company's products or technologies, including the products or formulations described in this release, or failure to obtain regulatory approval for the products or formulations, where required, or government regulations affecting AI or the AI algorithms not working as intended or producing accurate predictions; general economic conditions that are less favorable than expected; the FDA's general position regarding cannabis- and hemp-based products; and other factors, many of which are discussed in IGC Pharma's U.S. Securities and Exchange Commission ("SEC") filings. IGC incorporates by reference its Annual Report on Form 10-K filed with the SEC on June 24, 2024, and on Form 10-Qs filed with the SEC on August 7, 2024, November 12, 2024, and February 14, 2025, as if fully incorporated and restated herein. Considering these risks and uncertainties, there can be no assurance that the forward-looking information contained in this release will occur.
Contact Information:
Rosalyn Christian
IMS Investor Relations
[email protected]
(203) 972-9200
SOURCE: IGC Pharma, Inc.
View the original press release on ACCESS Newswire
M.Thompson--AMWN