
-
 US judge sets June 23 trial date over Boeing crashes
US judge sets June 23 trial date over Boeing crashes
-
S. Africa take big World Cup lead, but may lose points over Mokoena

-
 Zimbabwe moves army chief to sports docket
Zimbabwe moves army chief to sports docket
-
Stocks edge out gains as fears ease over next Trump tariffs

-
 'In my heart' - Malinin defends figure skating world title in wake of tragedy
'In my heart' - Malinin defends figure skating world title in wake of tragedy
-
Trump downplays firestorm over leaked Yemen air strike chat

-
 Turkey protesters fill streets, defying crackdown
Turkey protesters fill streets, defying crackdown
-
Roma's Dybala undergoes surgery on thigh injury

-
 US VP to visit Greenland as Trump ups pressure
US VP to visit Greenland as Trump ups pressure
-
What is Signal and is it secure?
-
 Political football as Iran reach World Cup while Australia, Saudis stay alive
Political football as Iran reach World Cup while Australia, Saudis stay alive
-
Brignone claims World Cup giant slalom title as Gut-Behrami wins finale

-
 UK artist Grayson Perry indulges playful side in new show
UK artist Grayson Perry indulges playful side in new show
-
Swiatek gets extra security after harassment

-
 Tuchel says Maguire 'will always be in contention' for England
Tuchel says Maguire 'will always be in contention' for England
-
Iran book World Cup spot as Australia, Saudis keep hopes alive

-
 Iran qualify for 2026 World Cup
Iran qualify for 2026 World Cup
-
Big bucks Iyer leads Punjab to win over Gujarat in IPL

-
 'Spider-Man,' 'Harry Potter' producers hired for new 007 film
'Spider-Man,' 'Harry Potter' producers hired for new 007 film
-
Trump, intel chiefs dismiss chat breach

-
 Boko Haram fighters kill 20 Cameroonian troops: sources
Boko Haram fighters kill 20 Cameroonian troops: sources
-
Bolsonaro headed 'criminal organization' to stay in power, court told

-
 Istanbul court jails 7 journalists as protesters fill streets
Istanbul court jails 7 journalists as protesters fill streets
-
Vernon takes Tour of Catalonia sprint as teen Brennan keeps lead

-
 Stocks meander as fears ease over next Trump tariffs
Stocks meander as fears ease over next Trump tariffs
-
Ex-Man City player Barton gets suspended jail term for assaulting wife

-
 UK judge slams Paddington Bear statue vandals
UK judge slams Paddington Bear statue vandals
-
Back in the pink: Senegal salt lake gets its colour back

-
 Robinson crashes out of World Cup giant slalom, Brignone eyes season title
Robinson crashes out of World Cup giant slalom, Brignone eyes season title
-
French art expert on trial over forged furniture at Versailles

-
 'An Italian miracle': Controversial Winter Olympics track slides into action
'An Italian miracle': Controversial Winter Olympics track slides into action
-
On US visit, Estonia warns of Putin 'upper hand' through talks

-
 Australia, Saudis keep World Cup hopes alive as S. Korea stutter again
Australia, Saudis keep World Cup hopes alive as S. Korea stutter again
-
Temple burned, UNESCO village evacuated as South Korea wildfires spread

-
 Lesotho's king warns nation will reel from Trump cuts
Lesotho's king warns nation will reel from Trump cuts
-
SpaceX rocket fuel makes stunning swirl in European sky

-
 US says Russia, Ukraine agree to end Black Sea military action
US says Russia, Ukraine agree to end Black Sea military action
-
EU unveils critical material projects to cut China dependence

-
 UK watchdog concerned Oasis fans 'misled' into buying costly tickets
UK watchdog concerned Oasis fans 'misled' into buying costly tickets
-
Barcelona basilica narrows down search for artist to design facade

-
 Brazil judges weigh whether to put Bolsonaro on trial for 'coup'
Brazil judges weigh whether to put Bolsonaro on trial for 'coup'
-
Faux gras? Scientists craft 'more ethical' version of French delicacy

-
 Turkish court jails 7 journalists after anti-Erdogan protests
Turkish court jails 7 journalists after anti-Erdogan protests
-
Trump brushes off Yemen chat breach as a 'glitch'

-
 Stocks up as fears ease over next Trump tariffs
Stocks up as fears ease over next Trump tariffs
-
Real Madrid making progress on Alexander-Arnold transfer: reports

-
 Depardieu denies 'groping' women in France sex abuse trial
Depardieu denies 'groping' women in France sex abuse trial
-
Olympic champion Ingebrigtsen testifies against father in abuse trial

-
 No Ukraine deal after US-Russia Saudi talks
No Ukraine deal after US-Russia Saudi talks
-
France to auction superyacht seized in money-laundering case


Tharimmune Announces Positive Results for Novel Oral Monoclonal Antibody TH023 Targeting Tumor Necrosis Factor-alpha
BRIDGEWATER, NJ / ACCESS Newswire / March 24, 2025 / Tharimmune, Inc. (Nasdaq:THAR) ("Tharimmune" or the "Company"), a clinical-stage biotechnology company focused on immunology and inflammation, today announced positive preclinical results for its novel oral antibody, TH023. In a murine model, a proprietary protease enzyme stabilized platform demonstrated successful delivery of infliximab, a tumor necrosis factor-alpha (TNF-α) inhibitor, in serum with concentrations detected being significantly higher than the standard serum trough concentration needed for antibody efficacy in immunology indications via injection (~3-5µg/ml). These findings represent a significant step towards developing a more convenient and potentially patient-preferred alternative to currently available infliximab treatments, which are administered via intravenous infusion or subcutaneous injection.
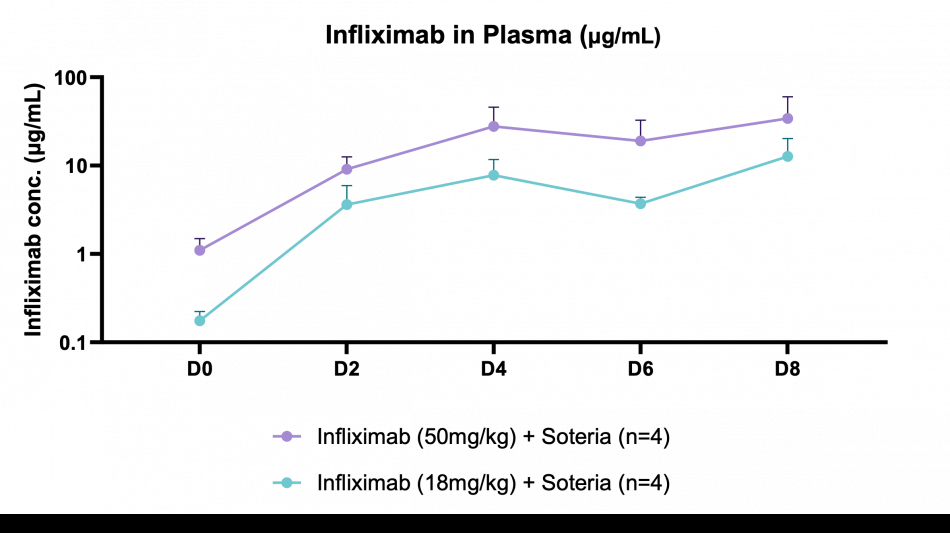
Key findings of the preclinical evaluation include demonstrating enzymatic protection of infliximab against human colon enzymes ex vivo using fresh fecal samples from healthy subjects utilizing the Soteria® platform, a proprietary formulation of natural amino acids (data not shown). Furthermore, successful delivery of TH023 in vivo into both local colonic tissue and systemic circulation was shown following intra-duodenal once-daily dosing for 1 week in a healthy mouse model at two doses of infliximab. This data shows the potential of the delivery platform to allow for both local delivery of the antibody precisely in the large intestinal tissue through enzymatic stabilization, as well as systemic circulation, which is an ideal pharmacokinetic (PK) profile for targeting both local gastrointestinal (GI) diseases such as inflammatory bowel disease (IBD) as well as systemic inflammatory diseases. The mechanism by which the antibody transcytosis occurs in the GI tract was shown to be a combination of passive, as well as mediated via the neonatal fragment crystallizable receptor (FcRn), highly expressed in distal intestinal epithelial cells enabling active transport.
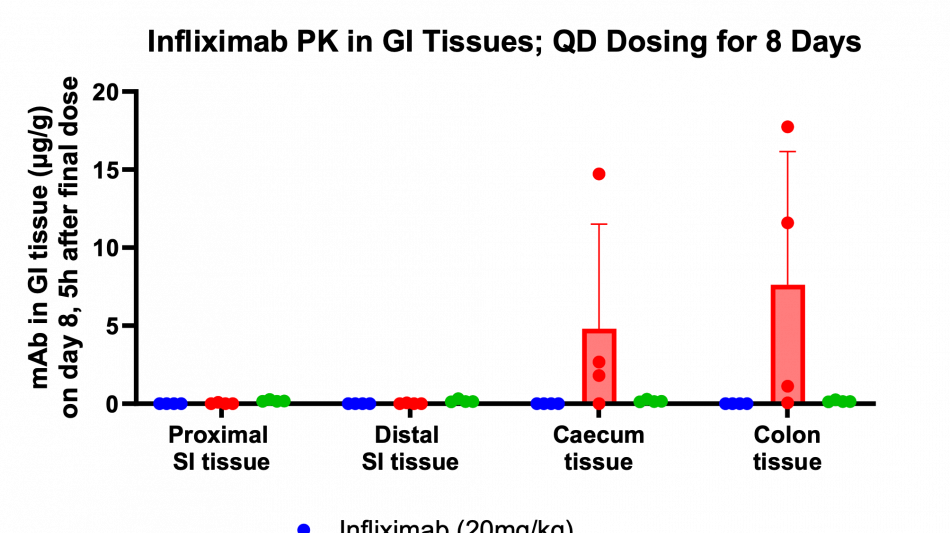
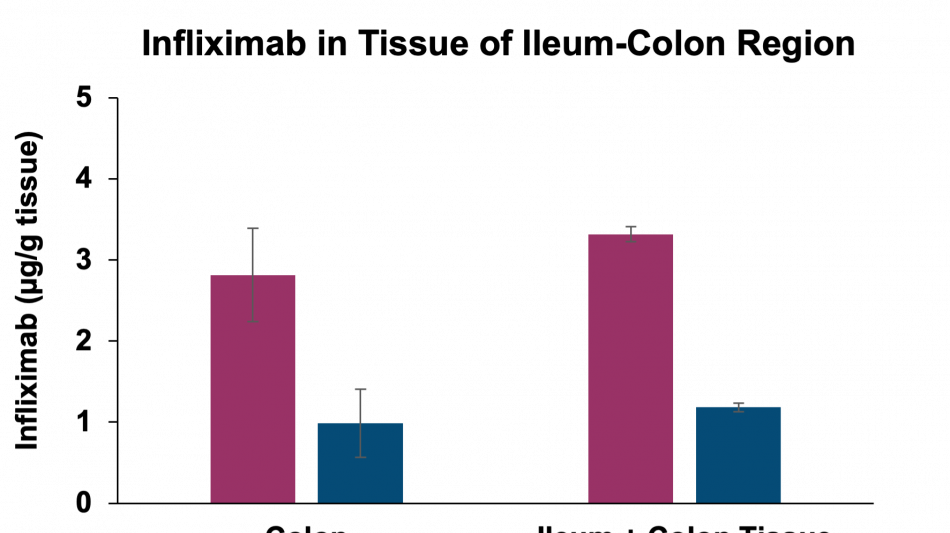
Additionally, the study demonstrated that tissue penetration of infliximab in combination with the enzyme stabilization platform was superior to a traditional permeation enhancer, sodium N-(8-[2-hydroxylbenzoyl] amino) caprylate (SNAC), which has been used to enhance the absorption of GLP-1 peptides, such as semaglutide. Utilization of SNAC to protect infliximab from enzymatic degradation or permeation enhancement did not result in tissue or serum concentrations suggesting standard off-the-shelf oral peptide delivery technologies are not suitable for oral delivery of antibodies. Two other standard permeation enhancer technologies tested (sodium caprate and labrasol) also showed unsitable results for oral delivery (data not shown), further supporting the Company's proprietary TH023 formulation.
The Company announced last year through a partnership with Intract Pharma, an exclusive license to INT-023 (now TH023), an oral anti-TNF-α monoclonal antibody. Tharimmune licensed global development and commercialization rights (outside of South Korea) to Intract Pharma's Soteria® and Phloral® delivery platform along with an existing supply agreement for infliximab to be used in the oral product development program. Traditionally administered through intravenous infusions, oral delivery of antibodies is challenging due to the complexity of navigating such large molecules through the GI tract. An oral route of administration holds potential to improve patient compliance and quality of life, while also reducing the burden on the healthcare system associated with long-term intravenous therapy.
"We are extremely encouraged by these results, which validate the potential of our partnership with Intract and the platform to deliver complex biologic molecules like infliximab orally," said Randy Milby, CEO of Tharimmune. "This represents a potential major milestone in our mission to develop more patient-friendly and accessible treatment options for chronic inflammatory diseases, addressing a multi-billion dollar market. These findings provide a strong foundation for further development into clinical trials."
Through the Company's existing partnership with Intract the data announced today enables for the targeted delivery of antibody therapeutics directly to the colon or small intestine. By leveraging Intract's platform, Tharimmune aims to enhance the effectiveness of TNF-α inhibitors such as infliximab through precision delivery that maximizes proteolytic stabilization and tissue permeation. This novel approach offers significant potential for directly addressing inflammatory conditions within the GI tract, including IBD as well as systemic inflammatory disorders where TNF-α plays a critical role in disease progression. Tharimmune plans to optimize the formulation and dosing regimen and prepare to conduct a first-in-human clinical trial with TH023 in the next 12 months.
Infliximab, a TNF-α inhibitor, is a widely used biologic for the treatment of several chronic inflammatory diseases, including Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. Globally, infliximab (including biosimilars) generated approximately $6.3 billion in sales in 2022, demonstrating the significant market for this therapy. However, current administration routes require frequent visits to healthcare facilities, which can be burdensome for patients and contribute to significant healthcare costs. Experts suggest the market for infliximab could rise to $9 billion within 10 years. An oral formulation of infliximab has the potential to improve patient convenience and compliance and eliminating the need for injections or infusions which could significantly improve the patient experience and potentially lead to better treatment adherence. Oral administration could reduce the need for clinic visits and specialized nursing care, potentially lowering overall treatment costs. A more convenient oral option could make infliximab accessible to a broader patient population, particularly in areas with limited access to infusion centers. By offering a differentiated, patient-preferred oral option, Tharimmune aims to capture a portion of the existing and growing infliximab market which represents a substantial commercial opportunity.
About Tharimmune, Inc.
Tharimmune is a clinical-stage biotechnology company developing a diverse portfolio of therapeutic candidates in immunology, inflammation and oncology. Its lead clinical asset, TH104, aims to suppress chronic pruritus associated with primary biliary cholangitis (PBC), a rare autoimmune liver disease with no known cure. The expanded pipeline includes TH023, an oral TNF-alpha inhibitor offering a new approach to treating autoimmune diseases. Tharimmune is also advancing early-stage multispecific biologics targeting unique epitopes against multiple solid tumors through its proprietary EpiClick™ Technology. The company has a license agreement with OmniAb, Inc. to access their antibody discovery technology for targeting specified disease markers. For more information, please visit: www.tharimmune.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, contained in this press release, including statements regarding the timing and design of Tharimmune's future Phase 2 trial, Tharimmune's strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words "anticipate," "believe," "continue," "could," "depends," "estimate," "expect," "intend," "may," "ongoing," "plan," "potential," "predict," "project," "target," "should," "will," "would," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that may cause such differences, include, but are not limited to, those discussed under Risk Factors set forth in our Annual Report on Form 10-K for the year ended December 31, 2023 and other periodic reports filed by the Company from time to time with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company's views as of the date of this release. Subsequent events and developments may cause the Company's views to change; however, the Company does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. These forward-looking statements should not be relied upon as representing the Company's views as of any date subsequent to the date of this release.
Contacts:
Tharimmune, Inc.
[email protected]
Alliance Advisors IR
Tirth T. Patel
[email protected]
212-201-6614
SOURCE: Tharimmune Inc.
View the original press release on ACCESS Newswire
G.Stevens--AMWN

