
-
 England thrash Scotland to set up France Grand Slam showdown
England thrash Scotland to set up France Grand Slam showdown
-
Verstappen's Red Bull 'comes alive' to claim record pole in Jeddah

-
 McTominay fires Napoli level with Inter as Conte fuels exit rumours
McTominay fires Napoli level with Inter as Conte fuels exit rumours
-
Rajasthan unleash Suryavanshi, 14, as youngest IPL player but lose thriller

-
 Man City boost top five bid, Aston Villa thrash in-form Newcastle
Man City boost top five bid, Aston Villa thrash in-form Newcastle
-
Villa rout Newcastle to rekindle bid to reach Champions League

-
 Dumornay gives Lyon lead over Arsenal in Women's Champions League semis
Dumornay gives Lyon lead over Arsenal in Women's Champions League semis
-
Trans rights supporters rally in London, Edinburgh after landmark ruling

-
 'We have to wait': Barca's Flick on Lewandowski injury fear
'We have to wait': Barca's Flick on Lewandowski injury fear
-
Bordeaux-Begles backups edge Pau to close in on Top 14 summit

-
 Trans rights supporters rally outside in London, Edinburgh after landmark ruling
Trans rights supporters rally outside in London, Edinburgh after landmark ruling
-
PSG beat Le Havre to stay on course for unbeaten Ligue 1 season

-
 Man City close in on Champions League with Everton late show
Man City close in on Champions League with Everton late show
-
14-year-old Vaibhav Suryavanshi becomes youngest IPL player

-
 Barca make stunning comeback to beat Celta Vigo in Liga thriller
Barca make stunning comeback to beat Celta Vigo in Liga thriller
-
Zverev sets up birthday bash with Shelton in Munich

-
 Man City boost top five bid, Southampton snatch late leveller
Man City boost top five bid, Southampton snatch late leveller
-
US Supreme Court intervenes to pause Trump deportations

-
 Alcaraz and Rune race into Barcelona final
Alcaraz and Rune race into Barcelona final
-
US, Iran to hold more nuclear talks after latest round

-
 Man City close in on Champions League thanks to Everton late show
Man City close in on Champions League thanks to Everton late show
-
Bayern close in on Bundesliga title with Heidenheim thumping

-
 Tunisia opposition figures get jail terms in mass trial
Tunisia opposition figures get jail terms in mass trial
-
Putin announces 'Easter truce' in Ukraine

-
 McLaren duo in ominous show of force in Saudi final practice
McLaren duo in ominous show of force in Saudi final practice
-
Afghan PM condemns Pakistan's 'unilateral' deportations

-
 Iran says to hold more nuclear talks with US after latest round
Iran says to hold more nuclear talks with US after latest round
-
Comeback queen Liu leads US to World Team Trophy win

-
 Buttler fires Gujarat to top of IPL table in intense heat
Buttler fires Gujarat to top of IPL table in intense heat
-
Unimpressive France stay on course for Grand Slam showdown

-
 Shelton fights past Cerundolo to reach Munich ATP final
Shelton fights past Cerundolo to reach Munich ATP final
-
Vance and Francis: divergent values but shared ideas

-
 Iran, US conclude second round of high-stakes nuclear talks in Rome
Iran, US conclude second round of high-stakes nuclear talks in Rome
-
Dumornay gives Lyon first leg lead over Arsenal in women's Champions League semis

-
 Trans rights supporters rally outside UK parliament after landmark ruling
Trans rights supporters rally outside UK parliament after landmark ruling
-
Rune destroys Khachanov to reach Barcelona Open final

-
 From Messi to Trump, AI action figures are the rage
From Messi to Trump, AI action figures are the rage
-
Vance discusses migration during Vatican meeting with pope's right-hand man

-
 Afghan FM tells Pakistan's top diplomat deportations are 'disappointment'
Afghan FM tells Pakistan's top diplomat deportations are 'disappointment'
-
British cycling icon Hoy and wife provide solace for each other's ills

-
 Money, power, violence in high-stakes Philippine elections
Money, power, violence in high-stakes Philippine elections
-
Iran, US hold second round of high-stakes nuclear talks in Rome

-
 Japanese warships dock at Cambodia's Chinese-renovated naval base
Japanese warships dock at Cambodia's Chinese-renovated naval base
-
US Supreme Court pauses deportation of Venezuelans from Texas

-
 Pakistan foreign minister arrives in Kabul as Afghan deportations rise
Pakistan foreign minister arrives in Kabul as Afghan deportations rise
-
Heat and Grizzlies take final spots in the NBA playoffs

-
 Iran, US to hold second round of high-stakes nuclear talks in Rome
Iran, US to hold second round of high-stakes nuclear talks in Rome
-
Humanoid robots stride into the future with world's first half-marathon

-
 Migrant's expulsion puts Washington Salvadorans on edge
Migrant's expulsion puts Washington Salvadorans on edge
-
Plan for expanded Muslim community triggers hope, fear in Texas


Tharimmune Announces Positive Target Engagement with Novel Biparatopic PD-1/VEGF and Multispecific HER2/HER3 Biologics Leveraging Proprietary EpiClick Technology
BRIDGEWATER, NJ / ACCESS Newswire / March 4, 2025 / Tharimmune, Inc. (NASDAQ:THAR) ("Tharimmune" or the "Company"), a clinical-stage biotechnology company focused on immunology and inflammation, today announced the expansion of its product pipeline with HS1940, a dual-target multispecific biologic engineered to bind to both Programmed Death-1 (PD-1) and Vascular Endothelial Growth Factor (VEGF) receptors. Using its proprietary EpiClick™ Technology, a versatile multispecific antibody engineering platform, HS1940 represents a key expansion of Tharimmune's product pipeline and underscores the Company's commitment to addressing unmet needs.
HS1940 is designed as a biparatopic (binding two epitopes on a single target) biologic, simultaneously engaging the PD-1 pathway and inhibiting angiogenesis. By using multiple, previously undruggable epitopes on PD-1, and blocking VEGF-mediated tumor vascularization, HS1940 may broaden treatment options and improve outcomes across multiple types of cancer and may access receptor regions that other PD-1 inhibitors (e.g., nivolumab and pembrolizumab) may not reach.
"We are extremely excited about the potential and versatility of our EpiClick platform. The implications for therapeutic intervention are potentially substantial, suggesting EpiClick-derived biologics may offer more potent and nuanced approaches to disease modulation," said Randy Milby, CEO of Tharimmune. "With EpiClick, we can strategically combine antibody binding domains to target multiple hard-to-reach epitopes, creating highly customized therapeutics tailored to the specifics of tumor destruction. For example, by addressing novel and multiple PD-1 epitopes while simultaneously inhibiting VEGF, we have the potential to offer a differentiated and more effective treatment option for a variety of solid tumors."
EpiClick enables the rapid and efficient creation of modular antibodies capable of high specificity and affinity toward multiple targets. A key feature of EpiClick is its "mix and match" approach, allowing distinct antibody binding domains - including those derived from previously inaccessible, undruggable epitopes - to be combined in either small-format or full-length configurations.
In the HS1940 family, the anti-PD-1 components leverage a novel design inspired by bovine antibodies, enabling them to target previously undruggable PD-1 epitopes. The anti-VEGF component, and the overall multispecific format, are engineered using EpiClick's modular design capabilities to create novel biparatopic PD-1 binding domains engineered on multiple VEGF scaffolds, as illustrated below.
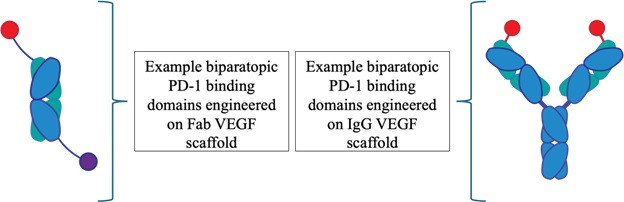
The Company has generated positive target engagement data showing that the biparatopic format expands therapeutic versatility compared with monospecific antibodies, which are limited to targeting a single epitope on a single receptor. As illustrated, the biparatopic antibody constructs effectively achieve target engagement on both PD-1 and VEGF. By engaging two distinct cellular pathways, HS1940 has the potential to elicit complex and synergistic anti-tumor effects. Tharimmune plans to continue preclinical testing with HS1940 and present data at future scientific conferences. The Company expects to initiate IND-enabling studies for HS1940 throughout 2025.
PD-1 is a well-validated immune checkpoint receptor that, when activated, suppresses T-cell function and allows cancer cells to evade immune detection. VEGF drives angiogenesis, which provides a nutrient and oxygen supply for tumors. By simultaneously blocking both pathways, HS1940 aims to achieve a synergistic anti-tumor effect by blocking PD-1, which releases immune "brakes," and enhancing T-cell-mediated tumor attack. Blocking VEGF disrupts tumor vasculature, starving them of nutrients.
Building upon the EpiClick platform, Tharimmune is also developing a new generation of multispecific antibodies targeting HER2 and HER3, two validated drivers of cancer growth and metastases. While HER2 is the focus of numerous successful commercial therapies, Tharimmune's approach offers distinct advantages. EpiClick leverages the "knob-and-stalk" from bovine-derived antibodies engineered to reach unique HER2 epitopes not addressed by existing drugs, while simultaneously engaging HER3. This dual engagement has the potential to disrupt cancer signaling in novel ways and overcome resistance to mechanisms associated with existing HER2-targeted therapies. By targeting distinct epitopes and incorporating HER3 engagement, Tharimmune's EpiClick-derived antibodies such as HS3215 offer a promising new avenue for more effective and targeted cancer treatments. Tharimmune is conducting preclinical studies to evaluate and optimize HS3215, with plans to advance the molecule into clinical trials following IND-enabling studies.
About EpiClick™ Technology
EpiClick™ Technology is a platform for creating customizable, multispecific antibodies that target previously undruggable epitopes, including those on PD-1, HER2, HER3 and other validated cancer targets. Inspired by bovine antibodies' unique "knob-and-stalk" structure, EpiClick uses engineered "knob" domains - small, precise binding units - that can "click" into recessed protein sites inaccessible to conventional antibodies. Its modular nature allows these "knobs," each targeting a specific epitope, to be paired with additional antibody components, creating a vast combinatorial library of multispecific therapeutics. For example, EpiClick enables the creation of novel biparatopic anti-PD-1 components, as used in HS1940, or HER2/HER3 domains, as in HS3215. By unlocking undruggable epitopes, EpiClick aims to deliver more effective and targeted treatments in immunotherapy and cancer therapy.
About Tharimmune, Inc.
Tharimmune is a clinical-stage biotechnology company developing a diverse portfolio of therapeutic candidates in immunology, inflammation and oncology. Its lead clinical asset, TH104, aims to suppress chronic pruritus associated with primary biliary cholangitis (PBC), a rare autoimmune liver disease with no known cure. The expanded pipeline includes TH023, an oral TNF-alpha inhibitor offering a new approach to treating autoimmune diseases. Tharimmune is also advancing early-stage multispecific biologics targeting unique epitopes against multiple solid tumors through its proprietary EpiClick™ Technology. The company has a license agreement with OmniAb, Inc. to access their antibody discovery technology for targeting specified disease markers. For more information, please visit: www.tharimmune.com.
Forward-Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, contained in this press release, including statements regarding the timing and design of Tharimmune's future Phase 2 trial, Tharimmune's strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words "anticipate," "believe," "continue," "could," "depends," "estimate," "expect," "intend," "may," "ongoing," "plan," "potential," "predict," "project," "target," "should," "will," "would," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that may cause such differences, include, but are not limited to, those discussed under Risk Factors set forth in our Annual Report on Form 10-K for the year ended December 31, 2023 and other periodic reports filed by the Company from time to time with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company's views as of the date of this release. Subsequent events and developments may cause the Company's views to change; however, the Company does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. These forward-looking statements should not be relied upon as representing the Company's views as of any date subsequent to the date of this release.
Contacts
Tharimmune, Inc.
[email protected]
Alliance Advisors IR
Tirth T. Patel
[email protected]
212-201-6614
SOURCE: Tharimmune Inc.
View the original press release on ACCESS Newswire
Ch.Havering--AMWN


