-
 Zelensky says Russian attacks ongoing despite Putin's Easter truce
Zelensky says Russian attacks ongoing despite Putin's Easter truce
-
Vaibhav Suryavanshi: the 14-year-old whose IPL dream came true

-
 Six drowning deaths as huge waves hit Australian coast
Six drowning deaths as huge waves hit Australian coast
-
Ukrainian soldiers' lovers kept waiting as war drags on

-
 T'Wolves dominate Lakers, Nuggets edge Clippers as NBA playoffs start
T'Wolves dominate Lakers, Nuggets edge Clippers as NBA playoffs start
-
Taxes on super rich and tech giants stall under Trump

-
 Star Wars series 'Andor' back for final season
Star Wars series 'Andor' back for final season
-
Neighbours improvise first aid for wounded in besieged Sudan city

-
 Tariffs could lift Boeing and Airbus plane prices even higher
Tariffs could lift Boeing and Airbus plane prices even higher
-
Analysts warn US could be handing chip market to China

-
 Unbeaten Miami edge Columbus in front of big MLS crowd in Cleveland
Unbeaten Miami edge Columbus in front of big MLS crowd in Cleveland
-
Social media helps fuel growing 'sex tourism' in Japan

-
 'Pandora's box': alarm bells in Indonesia over rising military role
'Pandora's box': alarm bells in Indonesia over rising military role
-
Alaalatoa hails 'hustling hard' Brumbies for rare Super Rugby clean sheet

-
 Trio share lead at tight LA Championship
Trio share lead at tight LA Championship
-
Sampdoria fighting relegation disaster as old heroes ride into town

-
 Recovering pope expected to delight crowds at Easter Sunday mass
Recovering pope expected to delight crowds at Easter Sunday mass
-
Nuggets edge Clippers in NBA playoff overtime thriller, Knicks and Pacers win

-
 Force skipper clueless about extra-time rules in pulsating Super Rugby draw
Force skipper clueless about extra-time rules in pulsating Super Rugby draw
-
Nuggets edge Clippers in NBA playoff overtime thriller, Pacers thump Bucks

-
 Unbeaten Miami edge Columbus in front of big crowd in Cleveland
Unbeaten Miami edge Columbus in front of big crowd in Cleveland
-
Kim takes one-shot lead over Thomas, Novak at RBC Heritage

-
 Another round of anti-Trump protests hits US cities
Another round of anti-Trump protests hits US cities
-
'So grateful' - Dodgers star Ohtani and wife welcome first child

-
 PSG maintain unbeaten Ligue 1 record, Marseille back up to second
PSG maintain unbeaten Ligue 1 record, Marseille back up to second
-
US, Iran report progress in nuclear talks, will meet again

-
 US Supreme Court intervenes to block Trump deportations
US Supreme Court intervenes to block Trump deportations
-
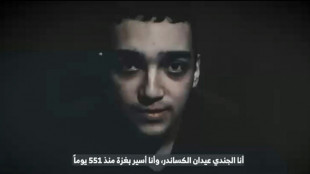
Hamas armed wing says fate of US-Israeli captive unknown
-
 Pacers thump Bucks to open NBA playoffs
Pacers thump Bucks to open NBA playoffs
-
Sabalenka reaches Stuttgart semis as Ostapenko extends Swiatek mastery

-
 Zelensky says Ukraine will observe Putin's Easter truce but claims violations
Zelensky says Ukraine will observe Putin's Easter truce but claims violations
-
'Fuming' Watkins fires Villa in bid to prove Emery wrong

-
 DR Congo boat fire toll revised down to 33
DR Congo boat fire toll revised down to 33
-
England thrash Scotland to set up France Grand Slam showdown

-
 Verstappen's Red Bull 'comes alive' to claim record pole in Jeddah
Verstappen's Red Bull 'comes alive' to claim record pole in Jeddah
-
McTominay fires Napoli level with Inter as Conte fuels exit rumours

-
 Rajasthan unleash Suryavanshi, 14, as youngest IPL player but lose thriller
Rajasthan unleash Suryavanshi, 14, as youngest IPL player but lose thriller
-
Man City boost top five bid, Aston Villa thrash in-form Newcastle

-
 Villa rout Newcastle to rekindle bid to reach Champions League
Villa rout Newcastle to rekindle bid to reach Champions League
-
Dumornay gives Lyon lead over Arsenal in Women's Champions League semis

-
 Trans rights supporters rally in London, Edinburgh after landmark ruling
Trans rights supporters rally in London, Edinburgh after landmark ruling
-
'We have to wait': Barca's Flick on Lewandowski injury fear

-
 Bordeaux-Begles backups edge Pau to close in on Top 14 summit
Bordeaux-Begles backups edge Pau to close in on Top 14 summit
-
Trans rights supporters rally outside in London, Edinburgh after landmark ruling

-
 PSG beat Le Havre to stay on course for unbeaten Ligue 1 season
PSG beat Le Havre to stay on course for unbeaten Ligue 1 season
-
Man City close in on Champions League with Everton late show

-
 14-year-old Vaibhav Suryavanshi becomes youngest IPL player
14-year-old Vaibhav Suryavanshi becomes youngest IPL player
-
Barca make stunning comeback to beat Celta Vigo in Liga thriller

-
 Zverev sets up birthday bash with Shelton in Munich
Zverev sets up birthday bash with Shelton in Munich
-
Man City boost top five bid, Southampton snatch late leveller


CB Scientific Inc. (CBSC) Formally Launches Next Generation MyCardia AT Cardiac Monitor
LAS VEGAS, NV / ACCESS Newswire / February 25, 2025 / CB Scientific, Inc. (OTC PINK:CBSC) ("CBSC" or the "Company"), a designer, manufacturer and distributor of non-invasive ambulatory cardiac monitoring products, announced today that the Company, in partnership with its contract manufacturer Datrix, LLC, is launching its next generation MyCardia AT cardiac event monitor targeted for use with our international distribution network in SE Asia, Hong Kong, and Macau. The newly branded MyCardia AT device is FDA-cleared and is designed to provide an improved patient remote cardiac monitoring experience with its lightweight, easy-to-wear design, including flexible and convenient options to transmit event recordings over an iOS or Android smartphone and utilizing our MyCardia Apple, Google or WeChat app.
The MyCardia AT cardiac event monitor helps physicians detect, identify, and diagnose potential cardiac arrhythmias in patients with intermittent transient symptoms closer to the time that they occur up to a 30-day monitoring period. Early detection and treatment of cardiac rhythm disorders helps to significantly reduce the burden of cardiac disease which can damage the heart and may even lead to an increased risk of stroke and death. According to the World Health Organization, since 2000 the largest increase in deaths is from ischemic heart disease which multiplied from 2.7 million to 9.1 million, and in 2021 accounted for 13% of the world's total deaths. The MyCardia AT device can also be used as an in-office ECG screening device.
The Company is strategically enhancing its remote cardiac monitoring portfolio with the launch of the MyCardia AT device. This initiative aims to leverage the strength of our established brand name to drive recognition, streamline our product ecosystem, and reinforce our market presence. The MyCardia AT marketing strategy involves seamlessly integrating with our existing AWS Cloud-based MyCardia portal and the widely adopted MyCardia iOS and Android smartphone applications, with the goal of ensuring a cohesive and optimized user experience on a global scale.
Our established distributors in Malaysia, Singapore, Thailand, Indonesia, Hong Kong, and Macau will now begin registering the MyCardia AT device with their respective regulatory agencies for import approval. Upon completion, the MyCardia AT device will begin shipping at that time and be widely available throughout these respective markets going forward. Additionally next step regulatory submission work will immediately resume for the MyCardia AT towards the approval of our representative Chinese manufacturing partner Shenzhen Pump. The Company will also continue working directly with representatives from the National Medical Products Administration (NMPA) in China towards the completion of clearance to market approval for our device into that market. Regulatory submission will also begin with our Canadian distributor Your Heart Protector Corp. towards clearance to market acceptance throughout Canada.
As additional new developments occur, CB Scientific, Inc. plans to make timely announcements through press releases and regulatory filings to keep its shareholders, industry participants, and the public markets informed.
About CB Scientific, Inc.
CB Scientific, Inc., through its international subsidiaries, provides innovative products and services in the ambulatory non-invasive cardiac monitoring space. Our electrocardiogram (EKG) devices, interactive cloud-based acquisition software, and smartphone apps for both iOS and Android platforms provide improved compliance for patients at risk of abnormal heart rhythms, as well as more accurate information for physicians.
Company Contact Information:
Telephone: (888) 225-0870
Email: General Inquiries: [email protected]
Investor Inquiries: [email protected]
Follow CBSC: X, Facebook, Instagram, LinkedIn, YouTube, and Newsletter
This information disclosure may contain forward-looking statements covered within the meaning of the Private Securities Litigation Act of 1995. These forward-looking statements relate to, among other things, plans and timing for the introduction or enhancement of our services and products, statements about future market conditions, supply and demand conditions, and other expectations, intentions, and plans contained in this press release that are not historical fact and involve risks and uncertainties. Our expectations regarding future revenues depend upon our ability to develop and supply products and services that we may not produce today and that meet defined specifications. When used in this press release, the words "plan," "expect," "believe," and similar expressions generally identify forward-looking statements. These statements reflect our current expectations. They are subject to a number of risks and uncertainties, including, but not limited to, changes in technology and changes in pervasive markets. This release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 27E of the Securities Act of 1934. Statements contained in this release that are not historical facts may be deemed to be forward-looking statements. Investors are cautioned that forward-looking statements are inherently uncertain. Actual performance and results may differ materially from that projected or suggested herein due to certain risks and uncertainties, including, without limitation, the ability to obtain financing and regulatory and shareholder approval for anticipated actions.
SOURCE: CB Scientific, Inc.
View the original press release on ACCESS Newswire
P.Santos--AMWN



