-
 England Test captain Stokes to miss early county games in fitness battle
England Test captain Stokes to miss early county games in fitness battle
-
Macron vows to defend science as host of UN oceans summit

-
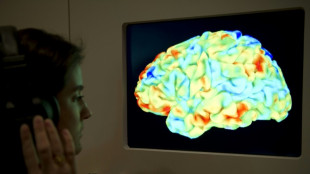 Brain implant turns thoughts into speech in near real-time
Brain implant turns thoughts into speech in near real-time
-
Top aide to Israel's Netanyahu arrested in 'Qatargate' probe

-
 Slashed US funding threatens millions of children: charity chief
Slashed US funding threatens millions of children: charity chief
-
China property giant Vanke reports annual loss of $6.8 bn

-
 World economies brace for Trump tariffs ahead of deadline
World economies brace for Trump tariffs ahead of deadline
-
Myanmar declares week of mourning as quake toll passes 2,000

-
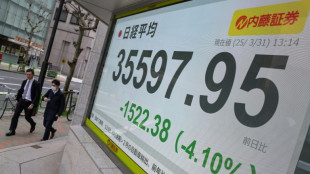 Japan leads hefty global stock market losses on tariff fears
Japan leads hefty global stock market losses on tariff fears
-
Yes, oui, Cannes! Glamour name eyes place in French Cup final

-
 'Different energy' at Man Utd after mini-revival, says Amorim
'Different energy' at Man Utd after mini-revival, says Amorim
-
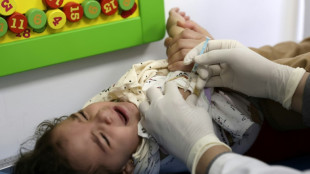
Fear of aftershocks in Myanmar forces patients into hospital car park

-
 Far-right leaders rally around France's Le Pen after election ban
Far-right leaders rally around France's Le Pen after election ban
-
Renault and Nissan shift gears on alliance

-
 Hard-hitting drama 'Adolescence' to be shown in UK schools
Hard-hitting drama 'Adolescence' to be shown in UK schools
-
Primark boss resigns after inappropriate behaviour allegation

-
 Myanmar declares week of mourning as quake toll passes 2,000, hopes fade for survivors
Myanmar declares week of mourning as quake toll passes 2,000, hopes fade for survivors
-
Mbappe can be Real Madrid 'legend' like Ronaldo: Ancelotti

-
 Saka 'ready to go' for Arsenal after long injury lay-off: Arteta
Saka 'ready to go' for Arsenal after long injury lay-off: Arteta
-
Aston Martin to sell stake in Formula One team

-
 Three talking points ahead of clay-court season
Three talking points ahead of clay-court season
-
French court hands Le Pen five-year election ban

-
 Probe accuses ex J-pop star Nakai of sexual assault
Probe accuses ex J-pop star Nakai of sexual assault
-
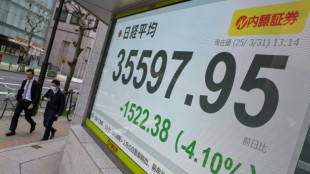
Japan leads hefty global stock market losses on tariff woes
-
 Saka 'ready to go' after long injury lay-off: Arteta
Saka 'ready to go' after long injury lay-off: Arteta
-
Ingebrigtsen Sr, on trial for abusing Olympic champion, says he was 'overly protective'

-
 Tourists and locals enjoy 'ephemeral' Tokyo cherry blossoms
Tourists and locals enjoy 'ephemeral' Tokyo cherry blossoms
-
Khamenei warns of 'strong' response if Iran attacked

-
 France fines Apple 150 million euros over privacy feature
France fines Apple 150 million euros over privacy feature
-
UK PM urges nations to smash migrant smuggling gangs 'once and for all'

-
 Thai authorities probe collapse at quake-hit construction site
Thai authorities probe collapse at quake-hit construction site
-
France's Le Pen convicted in fake jobs trial

-
 Chinese tech giant Huawei says profits fell 28% last year
Chinese tech giant Huawei says profits fell 28% last year
-
Trump says confident of TikTok deal before deadline
-
 Myanmar declares week of mourning as hopes fade for quake survivors
Myanmar declares week of mourning as hopes fade for quake survivors
-
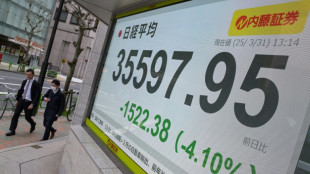
Japan's Nikkei leads hefty market losses, gold hits record
-
 Tears in Taiwan for relatives hit by Myanmar quake
Tears in Taiwan for relatives hit by Myanmar quake
-
Venezuela says US revoked transnational oil, gas company licenses

-
 'Devastated': Relatives await news from Bangkok building collapse
'Devastated': Relatives await news from Bangkok building collapse
-
Arsenal, Tottenham to play pre-season North London derby in Hong Kong

-
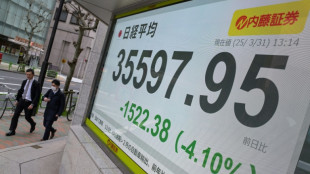 Japan's Nikkei leads hefty equity market losses; gold hits record
Japan's Nikkei leads hefty equity market losses; gold hits record
-
Israel's Netanyahu picks new security chief, defying legal challenge

-
 Trump says US tariffs to hit 'all countries'
Trump says US tariffs to hit 'all countries'
-
Prayers and tears for Eid in quake-hit Mandalay

-
 After flops, movie industry targets fresh start at CinemaCon
After flops, movie industry targets fresh start at CinemaCon
-
Tsunoda targets podium finish in Japan after 'unreal' Red Bull move

-
 French chefs await new Michelin guide
French chefs await new Michelin guide
-
UK imposes travel permit on Europeans from Wednesday

-
 At his academy, Romanian legend Hagi shapes future champions
At his academy, Romanian legend Hagi shapes future champions
-
Referee's lunch break saved Miami winner Mensik from early exit


US approves first pill for treatment of alopecia
The Food and Drug Administration on Monday approved a drug called baricitinib as the first oral tablet for treating severe alopecia areata, an autoimmune disorder affecting more than 300,000 people in the United States every year.
Alopecia causes either temporary or permanent patchy hair loss that can affect any hair-bearing site of the body, leading to emotional distress. The condition has come to the fore recently through high-profile cases including Hollywood actress Jada Pinkett Smith and congresswoman Ayanna Pressley.
"Access to safe and effective treatment options is crucial for the significant number of Americans affected by severe alopecia," said FDA official Kendall Marcus in a statement.
"Today's approval will help fulfill a significant unmet need for patients with severe alopecia areata."
Baricitinib, which is made by US pharmaceutical company Eli Lilly and known by the trade name Olumiant, belongs to a class of drugs called Janus kinase inhibitors. It works by interfering with the cellular pathway that leads to inflammation.
Its approval for use against alopecia was based on the results of two randomized, controlled clinical trials involving a total 1,200 adults with severe alopecia.
Each trial split participants into three groups: a placebo group, a group that received a two-milligram dose every day, and a group that received a four-milligram dose every day.
After 36 weeks, almost 40 percent of those on the higher dose grew back 80 percent of their scalp hair, compared to around 23 percent of the lower dose group, and five percent of the placebo group.
Around 45 percent of people in the higher dose group also saw significant eyebrow and eyelash regrowth.
The most common side effects included upper respiratory tract infections, headaches, acne, high cholesterol, and increase of an enzyme called creatinine phosphokinase.
Prior treatments for alopecia included topical or oral drugs, but these have been considered experimental and none was approved.
Baricitinib was previously approved for treatment of rheumatoid arthritis, and during the Covid pandemic its license was extended to the treatment of hospitalized Covid patients.
A.Jones--AMWN