-
 Iran delegation in Oman for high-stakes nuclear talks with US
Iran delegation in Oman for high-stakes nuclear talks with US
-
Australia beat Colombia to end BJK Cup bid on winning note

-
 German refinery's plight prompts calls for return of Russian oil
German refinery's plight prompts calls for return of Russian oil
-
Trump carves up world and international order with it

-
 Paris theatre soul-searching after allegations of sexual abuse
Paris theatre soul-searching after allegations of sexual abuse
-
US, Iran to hold high-stakes nuclear talks

-
 Frustrated families await news days after 222 killed in Dominican club disaster
Frustrated families await news days after 222 killed in Dominican club disaster
-
Jokic triple double as Denver fight back for big win

-
 Trump envoy suggests allied zones of control in Ukraine
Trump envoy suggests allied zones of control in Ukraine
-
Iraqi markets a haven for pedlars escaping Iran's economic woes

-
 Chinese manufacturers in fighting spirits despite scrapped US orders
Chinese manufacturers in fighting spirits despite scrapped US orders
-
Argentina receives $42 bn from international financial institutions

-
 Menendez brothers' resentencing can go ahead: LA judge rules
Menendez brothers' resentencing can go ahead: LA judge rules
-
'Hard on the body': Canadian troops train for Arctic defense

-
 Trump, 78, says feels in 'very good shape' after annual checkup
Trump, 78, says feels in 'very good shape' after annual checkup
-
McKellar 'very, very proud' after 'Tahs tame rampant Chiefs

-
 Man executed by firing squad in South Carolina
Man executed by firing squad in South Carolina
-
Defending champ Scheffler three back after tough day at Augusta

-
 Ballester apologizes to Augusta National for relief in Rae's Creek
Ballester apologizes to Augusta National for relief in Rae's Creek
-
Scorching Coachella kicks off as Lady Gaga set to helm main stage

-
 McIlroy, DeChambeau charge but Rose clings to Masters lead
McIlroy, DeChambeau charge but Rose clings to Masters lead
-
Langer misses cut to bring 41st and final Masters appearance to a close

-
 Ecuador presidential hopefuls make last pitch to voters
Ecuador presidential hopefuls make last pitch to voters
-
Rose knocking on the door of a major again at the Masters

-
 DeChambeau finding right balance at Augusta National
DeChambeau finding right balance at Augusta National
-
Spurs leaker not a player says Postecoglou

-
 All Black Barrett helps Leinster into Champions Cup semis
All Black Barrett helps Leinster into Champions Cup semis
-
Round-two rebound: Resilient McIlroy right back in the Masters hunt

-
 Asset flight challenges US safe haven status
Asset flight challenges US safe haven status
-
Menendez brothers appear in LA court for resentencing hearing

-
 McIlroy, DeChambeau charge as Rose clings to Masters lead
McIlroy, DeChambeau charge as Rose clings to Masters lead
-
UN seeks $275 million in aid for Myanmar quake survivors

-
 Frustrated families await news days after 221 killed in Dominican club disaster
Frustrated families await news days after 221 killed in Dominican club disaster
-
Trump wants to halt climate research by key agency: reports

-
 Fed official says 'absolutely' ready to intervene in financial markets
Fed official says 'absolutely' ready to intervene in financial markets
-
Slumping Homa happy to be headed into weekend at the Masters

-
 Morbidelli fastest ahead of cagey MotoGP title rivals in Qatar practise
Morbidelli fastest ahead of cagey MotoGP title rivals in Qatar practise
-
Musetti stuns Monte Carlo Masters champion Tsitsipas to reach semis

-
 Abuse scandal returns to haunt the flying 'butterflies' of Italian gymnastics
Abuse scandal returns to haunt the flying 'butterflies' of Italian gymnastics
-
Trump defends policy after China hits US with 125% tariffs

-
 Frustrated families await news days after Dominican club disaster
Frustrated families await news days after Dominican club disaster
-
McLarens dominate Bahrain practice, Verstappen rues 'too slow' Red Bull

-
 Eight birdies rescue Masters rookie McCarty after horror start
Eight birdies rescue Masters rookie McCarty after horror start
-
RFK Jr's autism 'epidemic' study raises anti-vaxx fears

-
 Trump -- oldest elected US president -- undergoes physical
Trump -- oldest elected US president -- undergoes physical
-
Rose clings to Masters lead as McIlroy, DeChambeau charge

-
 Brazil's Bolsonaro hospitalized with abdominal pain, 'stable'
Brazil's Bolsonaro hospitalized with abdominal pain, 'stable'
-
Canada, US to start trade talks in May: Carney

-
 Six arrested for murder of notorious Inter Milan ultra
Six arrested for murder of notorious Inter Milan ultra
-
Pig kidney removed from US transplant patient, but she set record


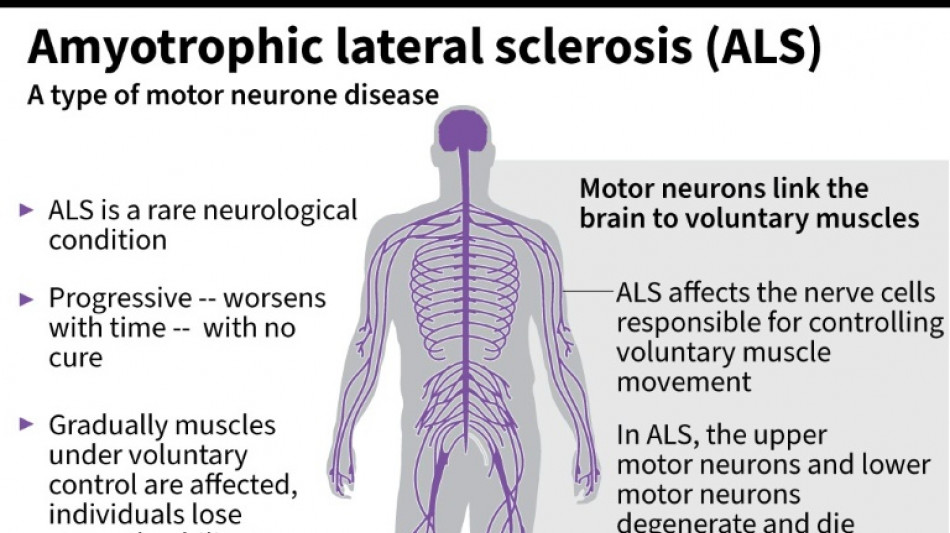
Every month counts: European ALS patients want new drugs
Olivier Goy is running out of time.
The French entrepreneur was diagnosed in 2020 with amyotrophic lateral sclerosis (ALS) -- the incurable neurodegenerative disease that normally claims the lives of patients within three to five years.
There are new treatments that have given patients hope of being able to extend their lives by an invaluable few months, but the approval process in Europe is taking time, infuriating desperate patients.
"When you are certain to die soon, patients and some doctors are ready to take some risks," Goy told AFP.
In response to the lack of new treatments in his native France, the founder of the fintech start-up October spends 3,000 euros ($3,180) every month to buy the ingredients to make his own drugs.
ALS, also known as Lou Gehrig's disease, attacks the motor nerve cells in the brain and spinal cord, progressively paralysing muscles until patients cannot walk, eat, speak or breathe.
Around one in 10,000 people have the disease in the EU, according to the European Medicines Agency.
The drug Riluzole, which has been available in Europe and the UK since the 1990s, is capable of prolonging the lives of patients by around three months.
But otherwise, no new treatment has been approved in Europe for more than two decades.
- 'First hope in 20 years' -
A new treatment called AMX0035 was given the green light in the United States and Canada last year.
"It is the first hope we have had in 20 years: the first drug which is aimed at everyone and which had results" suggesting up to six months in added life expectancy, said Sabine Turgeman, head of the French Association for Research into ALS.
But the extent of the benefits of AMX0035 remains unclear. The US Food and Drug Administration approved the drug, sold under the name Relyvrio, based on the results of a single Phase 2 trial that involved just 137 participants.
The drug's developer, Amylyx Pharmaceuticals, is conducting larger, more comprehensive trials, with results expected in 2024.
Amylyx said earlier this month that the European Union's drug watchdog EMA is reviewing its submission for approval and it expects a decision in the first half of this year.
But for those with the disease, every delay represents a significant amount of the time they have left.
"It's not going fast enough," Turgeman said. "This disease is not on bureaucratic time".
For European patients who cannot afford to import their own ingredients like Goy, the only way to get access to new treatments is to join a clinical trial.
But such trials have very specific criteria for selection -- and even if a patient gets in, there is a chance they will be in the group given a placebo.
- 'Totally abandoned' -
Given how swiftly the disease progresses, patients and families are pressing for more options.
"We feel totally abandoned," said Sophie Garofalo, whose brother was diagnosed with ALS five years ago.
His family tried to enter him into clinical trials, "but either he does not meet the criteria, or the trials have already started," she said.
"He is ready to take anything, try everything".
French pharmaceutical company AB Science is developing another potential treatment using the drug masitinib, which initial results suggest could add months to the lives of patients.
The firm's CEO Alain Moussy said that because "time is very limited" for ALS patients, there should be more flexibility in the approval system.
"What degree of risk should be taken? That's for the health agencies to answer -- but they can guided by policymakers and patients," he said.
O.M.Souza--AMWN