
-
 Stocks rise on bank earnings, auto tariff hopes
Stocks rise on bank earnings, auto tariff hopes
-
Trump showdown with courts in spotlight at migrant hearing

-
 Ecuador electoral council rejects claims of fraud in presidential vote
Ecuador electoral council rejects claims of fraud in presidential vote
-
Russia jails four journalists who covered Navalny

-
 Trump says China 'reneged' on Boeing deal as tensions flare
Trump says China 'reneged' on Boeing deal as tensions flare
-
Trump eyes near 50 percent cut in State Dept budget: US media

-
 Trump says would 'love' to send US citizens to El Salvador jail
Trump says would 'love' to send US citizens to El Salvador jail
-
'Unprecedented' Europe raids net 200 arrests, drugs haul

-
 Everyone thinks Real Madrid comeback 'nailed-on': Bellingham
Everyone thinks Real Madrid comeback 'nailed-on': Bellingham
-
NATO's Rutte says US-led Ukraine peace talks 'not easy'

-
 Harvey Weinstein New York retrial for sex crimes begins
Harvey Weinstein New York retrial for sex crimes begins
-
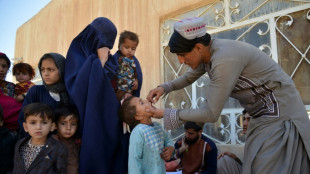
More than 10% of Afghans could lose healthcare by year-end: WHO
-
 Stocks rise as auto shares surge on tariff break hopes
Stocks rise as auto shares surge on tariff break hopes
-
Facebook chief Zuckerberg testifying again in US antitrust trial

-
 Pakistan court refuses to hear Baloch activist case: lawyers
Pakistan court refuses to hear Baloch activist case: lawyers
-
Inzaghi pushing Inter to end San Siro hoodoo with Bayern and reach Champions League semis

-
 Arsenal's Odegaard can prove point on Real Madrid return
Arsenal's Odegaard can prove point on Real Madrid return
-
China's Xi begins Malaysia visit in shadow of Trump tariffs

-
 Andrew Tate accusers suing for 'six-figure' sum, UK court hears
Andrew Tate accusers suing for 'six-figure' sum, UK court hears
-
Macron to honour craftspeople who rebuilt Notre Dame

-
 Van der Poel E3 'spitter' facing fine
Van der Poel E3 'spitter' facing fine
-
Khamenei says Iran-US talks going well but may lead nowhere

-
 Nearly 60,000 Afghans return from Pakistan in two weeks: IOM
Nearly 60,000 Afghans return from Pakistan in two weeks: IOM
-
Auto shares surge on tariff reprieve hopes

-
 Sudan war drains life from once-thriving island in capital's heart
Sudan war drains life from once-thriving island in capital's heart
-
Trump trade war casts pall in China's southern export heartland

-
 Ukraine's Sumy prepares to bury victims of 'bloody Sunday'
Ukraine's Sumy prepares to bury victims of 'bloody Sunday'
-
Iraq sandstorm closes airports, puts 3,700 people in hospital

-
 French prisons targeted with arson, gunfire: ministry
French prisons targeted with arson, gunfire: ministry
-
Pandemic treaty talks inch towards deal

-
 Employee dead, client critical after Paris cryotherapy session goes wrong
Employee dead, client critical after Paris cryotherapy session goes wrong
-
Howe will only return to Newcastle dugout when '100 percent' ready

-
 Journalist recalls night Mario Vargas Llosa punched Gabriel Garcia Marquez
Journalist recalls night Mario Vargas Llosa punched Gabriel Garcia Marquez
-
Sudan marks two years of war with no end in sight

-
 Vance urges Europe not to be US 'vassal'
Vance urges Europe not to be US 'vassal'
-
China tells airlines to suspend Boeing jet deliveries: report

-
 Stocks rise as stability returns, autos surge on exemption hope
Stocks rise as stability returns, autos surge on exemption hope
-
Harvard sees $2.2bn funding freeze after defying Trump
-
 'Tough' Singapore election expected for non-Lee leader
'Tough' Singapore election expected for non-Lee leader
-
Japan orders Google to cease alleged antitrust violation

-
 Stocks rise as stability returns, autos lifted by exemption hope
Stocks rise as stability returns, autos lifted by exemption hope
-
Malawi's debt crisis deepens as aid cuts hurt

-
 Danish brewer adds AI 'colleagues' to human team
Danish brewer adds AI 'colleagues' to human team
-
USAID cuts rip through African health care systems

-
 Arsenal target Champions League glory to save season
Arsenal target Champions League glory to save season
-
Kane and Bayern need killer instinct with home final at stake

-
 Mbappe leading Real Madrid comeback charge against Arsenal
Mbappe leading Real Madrid comeback charge against Arsenal
-
S. Korea plans extra $4.9 bn help for chips amid US tariff anxiety

-
 Xi's Vietnam trip aiming to 'screw' US, says Trump
Xi's Vietnam trip aiming to 'screw' US, says Trump
-
Iran's top diplomat to visit Russia after US nuclear talks

| RBGPF | 0.22% | 63.59 | $ | |
| CMSC | -0.11% | 21.785 | $ | |
| BCC | -1.19% | 93.79 | $ | |
| SCS | -2.66% | 9.965 | $ | |
| BTI | 0.98% | 42.425 | $ | |
| GSK | 1.25% | 35.725 | $ | |
| NGG | 1.62% | 70.53 | $ | |
| RYCEF | 3.4% | 9.71 | $ | |
| RIO | 0.92% | 57.54 | $ | |
| CMSD | 0.23% | 21.96 | $ | |
| RELX | 2.69% | 51.507 | $ | |
| BCE | -1.91% | 21.245 | $ | |
| JRI | 1.51% | 12.18 | $ | |
| AZN | -0.35% | 67.775 | $ | |
| VOD | 1.7% | 9.115 | $ | |
| BP | 1.91% | 27.435 | $ |

DEA Ignored Marijuana Federal Law? MMJ's 7 Year Delay on Critical Drug Development for Suffering Patients
DEA Marijuana Seven-Year Delay in MMJ Biopharma Cultivation’s Application Sparks Calls for DEA Accountability and Reform "This isn't just a case of bureaucratic red tape or administrative lag-this appears to be a deliberate disregard for the well-being of patients and a blatant violation of federal law," asserted Duane Boise, CEO of MMJ Biopharma Cultivation. "Every year of delay in this type of critical research can cost lives and prolong the suffering of countless individuals."
WASHINGTON, DC / ACCESS Newswire / April 14, 2025 / Senior officials at the Drug Enforcement Administration (DEA), THOMAS PREVOZNIK, MATTHEW STRAIT AND AARTHI HAIG are facing mounting scrutiny following explosive allegations that they deliberately obstructed a critical medical marijuana research application for nearly a decade, brazenly flouting federal law, presidential directives, and the agency's own stated mission to support legitimate scientific pharmaceutical development needs. The eye of this storm centers on MMJ Biopharma Cultivation, a pioneering pharmaceutical company that sought the necessary permissions to cultivate specialized cannabis strains for FDA-approved clinical trials aimed at developing desperately needed treatments for Huntington's Disease and Multiple Sclerosis (MS)-only to be met with a staggering seven-year delay in the approval process.

The Backstory: DEA Seven-Year Black Hole of Bureaucracy
The ordeal began in 2018 when MMJ Biopharma Cultivation submitted its application for a DEA bulk manufacturing registration (grow pharmaceutical marijuana). The company's objective was clear and scientifically driven: to cultivate specific marijuana cultivars under strict pharmaceutical-grade standards for use in rigorous clinical trials. These trials held the promise of developing innovative therapies for Huntington's Disease, a devastating and currently incurable neurodegenerative disorder, and MS, a debilitating autoimmune condition affecting over a million individuals in the United States alone.
Despite the profound implications for medical science and the urgent needs of patients suffering from these diseases, MMJ Biopharma Cultivation's application languished within the DEA's bureaucratic labyrinth, remaining in limbo to date. This protracted delay has ignited numerous law suits, outrage among patient advocacy groups, medical researchers, and lawmakers, who decry it as "unconscionable" and "legally indefensible."
The staggering hold-up directly contravenes the Medical Marijuana and Cannabidiol Research Expansion Act (H.R.8454), a bipartisan bill signed into law in December 2022. This landmark legislation explicitly mandates that the DEA "expedite approvals" for marijuana research applications within a strict 60-day timeframe. Furthermore, the delay appears to be a direct affront to the DEA's own Diversion Control Division mission, which includes the critical responsibility of ensuring "an uninterrupted supply of controlled substances for legitimate medical and scientific needs."
DEA Key Players and Damning Allegations
Internal memos and accounts obtained by unnamed sources have identified three senior DEA officials who allegedly played pivotal roles in the prolonged obstruction of MMJ Biopharma Cultivation's application:
Matthew Strait, Deputy Assistant Administrator of the Diversion Control Division, is alleged to have consistently prioritized bureaucratic hurdles and internal procedures over the urgent public health imperatives of facilitating critical medical research.
Aarthi Haig, Associate Chief Counsel in the DEA's Office of Legal Counsel, is accused of providing legal justifications for the years-long delays, seemingly disregarding the clear statutory obligations outlined in H.R.8454.
"This isn't just a case of bureaucratic red tape or administrative lag-this appears to be a deliberate disregard for the well-being of patients and a blatant violation of federal law," asserted Duane Boise, CEO of MMJ Biopharma Cultivation. "Every year of delay in this type of critical research can cost lives and prolong the suffering of countless individuals."
DEA Legal and Ethical Minefield
Critics contend that the DEA officials' alleged actions constitute a profound breach of public trust and raise serious legal and ethical concerns:
Blatant Violation of Federal Law: H.R.8454 unequivocally requires the DEA to act on legitimate marijuana research applications within a 60-day window. MMJ Biopharma's agonizing seven-year wait strongly suggests a systemic pattern of noncompliance within the agency.
Contempt for Presidential Priorities: President Biden, in a significant move in 2022, issued an executive order directing federal agencies to streamline the process for marijuana research under the Controlled Substances Act. The alleged obstruction appears to directly undermine these White House directives.
Fundamental Undermining of the DEA's Core Mission: The DEA's Diversion Control Division is tasked with the delicate balancing act of preventing the diversion of controlled substances while simultaneously ensuring their availability for legitimate medical and scientific purposes. Advocates argue that the agency's handling of MMJ Biopharma Cultivation's application represents a clear failure on both fronts.
"This is a textbook case of bureaucratic inertia and potential malfeasance tragically overriding basic human compassion and the urgent needs of the scientific community," declared Duane Boise. In H.R.8454. "Congress specifically wrote this law to put an end to these exact kinds of inexcusable delays. The DEA's apparent defiance of this clear legislative mandate cannot and must not go unchecked."
The Crushing Human Cost of DEA'S Bureaucratic Indifference
For patients like 34-year-old Jessica Marlow, who received a devastating diagnosis of Huntington's Disease in 2020, the DEA's years of delay are not abstract bureaucratic failures-they are deeply personal and fraught with despair. "There are currently no effective treatments to slow the relentless progression of this horrific disease," she shared, her voice filled with palpable frustration and anguish. "The type of groundbreaking research that MMJ was trying to conduct offers a glimmer of hope, a potential pathway to a future where we might not have to watch our lives slowly disappear. But with each passing year of bureaucratic delay, that hope dwindles, and for many of us, time is a luxury we simply don't have."
Huntington's Disease, a cruel genetic disorder, relentlessly destroys nerve cells in the brain, leading to a progressive decline in physical, mental, and emotional abilities, ultimately resulting in death typically within 10 to 20 years after diagnosis. Multiple Sclerosis, while not always fatal, causes irreversible damage to the protective sheath surrounding nerve fibers, disrupting communication between the brain and the body, leading to a wide range of debilitating symptoms that can rob patients of their mobility, independence and overall quality of life.
Mounting Calls for DEA Accountability and Systemic Reform
A growing chorus of watchdog organizations, patient advocacy groups, and bipartisan lawmakers are demanding swift and decisive action to address the alleged obstruction and prevent future occurrences:
Immediate Approval: Pressure is mounting on the DEA to immediately and retroactively grant MMJ BiopharmaCultivation's long-pending application to salvage the stalled and critically important clinical trials.
Thorough DOJ Investigation: The Department of Justice's Office of the Inspector General (OIG) is being urgently called upon to launch a comprehensive probe into potential misconduct and systemic issues within the DEA that may have contributed to the egregious delays.
Congressional Hearings: Key House oversight committees are likely to summon DEA leadership to appear before Congress and provide a detailed explanation for the years of delays and apparent disregard for federal law.
Comprehensive Policy Reforms: Advocates are pushing for the implementation of mandatory strict deadlines for application processing, transparent accountability metrics for DEA officials, and enhanced personnel training to ensure future compliance with legislative mandates and the agency's core mission.
The DEA declined to offer specific comments on ongoing litigation related to MMJ Biopharma's case.
A Potential Turning Point for Federal Drug Policy?
The unfolding MMJ Biopharma Cultivation's saga has reignited a fierce debate over the DEA's appropriate role in an era of rapidly shifting drug policies. With 38 states having legalized medical marijuana and a growing bipartisan consensus supporting the expansion of cannabis research, critics argue that the agency's operational culture remains deeply entrenched in outdated prohibitionist tactics that actively impede scientific progress and patient access.
"This isn't simply about the complexities of cannabis regulation-this case cuts to the heart of whether the DEA, as a federal agency, ultimately serves the interests of public health and scientific advancement or remains tethered to outdated political ideologies and bureaucratic inertia," emphasized Duane Boise, MMJ Biopharma Cultivation CEO, an advocate for cannabis policy reform. "The level of accountability demanded and achieved in this case could potentially reshape federal drug policy for decades to come."
What Lies Ahead?
The Department of Justice is reportedly expected to ACT within the coming weeks regarding launching a formal investigation into the actions of the named DEA officials.
MMJ Biopharma Cultivation has indicated its intention to pursue legal action against the DEA, seeking significant damages, should its application not be granted immediate approval.
Patient advocates and policy reform organizations have vowed to intensify pressure on the TRUMP administration and DOGE to undertake a comprehensive overhaul of DEA leadership and to re-evaluate the federal marijuana policy in light of its recognized medical potential and the clear directives of Congress.
For the countless patients and dedicated researchers who have waited with bated breath for progress in this critical field, the resounding message is clear: After seven long years of bureaucratic obstruction, justice for patients and the rule of law cannot wait any longer.
MMJ is represented by attorney Megan Sheehan.
CONTACT:
Madison Hisey
[email protected]
203-231-8583
Related Links:
SOURCE: MMJ International Holdings
View the original press release on ACCESS Newswire
X.Karnes--AMWN